EU Regulation 2017/745 (MDR) states that product information must be “steered”. This is very important for the fulfillment of ISO 13485.
DIN EN ISO 13485 is a quality management system for compliance with the manufacturing and placing on the market of medical devices. The requirement of EN ISO 13485: 2016, chapter 4.2. "Document control" is a particular challenge for companies that are active in medical technology.
This chapter includes - especially in the future - the "control of website content". EU Regulation 2017/745 (MDR), which will come into force on May 26, 2021, shows that product information must also be “controlled”.
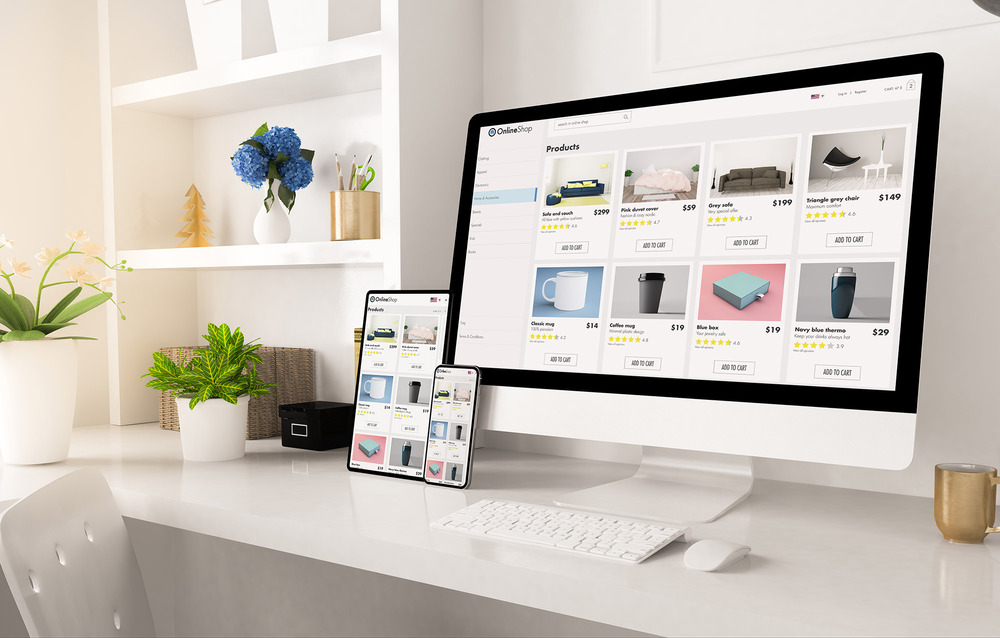
In the field of medical technology, product information means: instructions for use, certificates, clinical data, marketing materials and application descriptions that are based on the technical documentation.
The SQ QM Web Documentation therefore documents:
- Who has when and what content on the website
- created , checked , released and changed.
You can find more information on our website.
To the original article