
The manufacture of needle holders is also one of our core competencies. Our needle holders are manuf...
Portal and digital medical technology fair of the largest MedTech cluster in Germany

The manufacture of needle holders is also one of our core competencies. Our needle holders are manuf...

Surgical instruments Millions of surgeons throughout the world use and rely on our surgical instr...

High quality, medical, flexible ultrasound...
Our product range of medical endoscopes - High quality, medical, flexible ultrasound endoscopes...
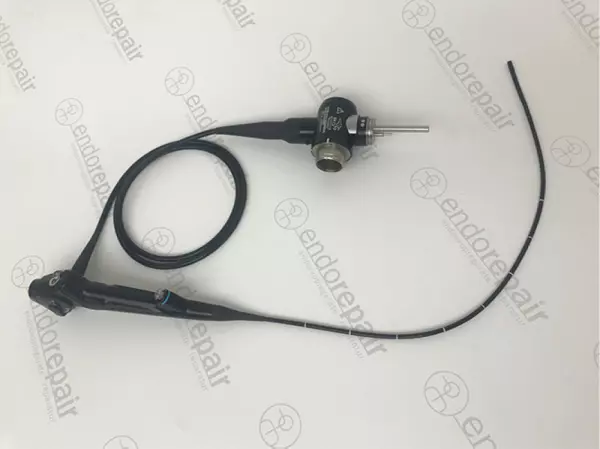
High quality, medical, flexible bronchoscope...
Our product range of medical endoscopes - High quality, medical, flexible bronchoscope for expe...

Micro needle holders, scissors and forceps are required for modern and state of the art micro discip...

Tubular shaft instruments for conservative and minimally invasive surgery made by STRUB MEDICAL are...

Certified repair services of ultrasonic probes...
endorepair has evolved in the last 2 years and prepared another repair branch. During the two q...
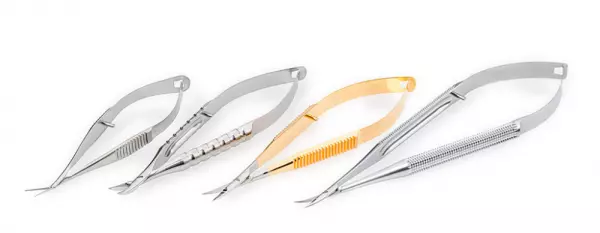
Cutting spring-handled instruments
MICRO INSTRUMENTS - 100% MADE IN GERMANY Below you will find an excerpt from our product portfoli...

High quality, medical, flexible Coloscopes...
Our product range of medical endoscopes - High quality, medical, flexible Coloscopes for expert...

Repair service provider for your endoscopy...
endorepair – your partner for the repair of your endoscopy devices and ultrasound probes Ve...

Microsurgical instruments in the premium...
OPHTHALMOLOGY WE MANUFACTURE MICRO-EYE INSTRUMENTS FOR THE FOLLOWING INTERVENTIONS: ...

Various surgical instruments We offer standard and custom designs for a wide variety of applicati...

Plastic Surgery Instruments - TC Forceps
PLASTIC SURGERY In plastic surgery, interventions are performed for cosmetic as well as for funct...

Bypassing repairs and rental equipment for...
At endorepair, we help you minimise your economic downtime. We achieve this on the one hand through...
Plastic surgery is a sub-area of surgery and deals with the change of organs or the skin surface for aesthetic or functional reasons. Aesthetic surgery includes correction of protruding ears, breast enlargement or reduction, tightening of the skin, liposuction, surgery of varicose veins or the removal of skin tumors.
Burns are one of the most serious types of injuries that the skin and the underlying tissue layers can suffer and are also part of plastic surgery. In burn surgery, specialists work with special skin shifting techniques or with transplants to remove traces of flat burns.
Aesthetic Surgery has a long history. Some of the first aesthetic surgeries were performed in the early 20th century to correct a patient's protruding ears. The field was primarily focused on the face and ears until C.C. Miller began writing books on the subject. Before world war I, aesthetic surgery was rarely practiced. But the advent of the military and increased demand for facial enhancements spawned several maxillo-facial surgery units.
APOCs regulate the practices of aesthetic surgeons, and their adherence to the SMC's ethical guidelines and code of ethics is critical to the practice of medical aesthetics. Aesthetic procedures are not always approved by the SMC, and doctors must be able to demonstrate technical skill and competence before performing them. Patients should always be informed before any surgical procedure, and doctors must ensure patient safety at all times. To avoid malpractice claims, medical practitioners should register under the SMC's APOC.
The SMC requires that cosmetic surgeons have completed a minimum number of cosmetic procedures to meet eligibility requirements. The SMC has a strict set of criteria for eligibility, including a minimum number of aesthetic cases. In the case of nonsurgical procedures, a doctor must meet these criteria or risk losing his/her membership in the Society. The SMC's ethical code states that the SMC must ensure that doctors have the necessary knowledge, technical skills and training to perform such procedures.
Cosmetics are regulated by the Food and Drug Administration and are not performed by physicians. However, the FDA and EU regulate aesthetic procedures, so cosmetic products are often confused with medicine. There are also risks associated with using medical devices to treat cosmetic issues. Fortunately, governments are doing their part to educate consumers. Moreover, they are requiring manufacturers to include instructions on the label. But if you're looking to use aesthetic devices, the best way is to consult an expert in this field.
The FDA has strict regulations for aesthetic devices. As a result, a device must be classified according to its intended use and risk. This makes it easy for a manufacturer to get the product on the market. The FDA also requires cosmetic devices to be marketed appropriately. Unlike other medical devices, they are not subject to the same regulations and standards as medical products. In fact, the FDA does not even know if a device is considered a medical device.
There are a few types of aesthetic devices. Most of them are based on four basic principles. There are four groups of products: home use aesthetic products, medical devices, and cosmetic procedures. For a more detailed discussion of these terms, visit the ASAPS website. They also have a list of the products they are regulating. They can't be used without the consent of a physician, which is required for the safety of patients.
Become a digital exhibitor yourself in the online portal of the largest and best-known MedTech cluster region in Germany and inform the world of medical technology about your products and services as well as about news, events and career opportunities.
With an attractive online profile, we will help you to present yourself professionally on our portal as well as on Google and on social media.